Connection of humic substances with mineral fraction
- Extensive studies have shown that not much of the humic substances in soil is in free state
- but much is bound to colloidal clay.
- The ways in which humic substances are combination with mineral portion of soil as
- follows:
- As salts of low - molecular organic acids (acetate, oxalate, lactate and others).
- As salts of humic substances with alkaline cations - humate, fulvate.
- As chelate with metal ions.
- As substances held on clay mineral surfaces.
Salts of low - molecular organic acids
- Salts of low - molecular acids forms in result of action of acids (acetic acid, oxalic acid,
- fumaric acid, lactic acid) on minerals (magnesite, calcite, siderite and others) or salts of mineral acids with Ca, K and others cations.
Salts of humic substances with alkaline cations
- Salts of humic substances with alkaline cations comprehensive of compounds:
-
- humate (salts of humic acids)
- fulvate (salts of fulvic acids)
- They are the most characteristic compounds of soil humic substances.
The alkaline
- cations (Na+, K+, Ca2+, Mg2+) are held primarily by simple cation exchange with COOH groups (RCOONa, RCOOK etc.).
The humate and fulvate occur in soil largely as mixture with hydroxide of Fe and Al.
Chelate with metal ions
- A
chelate complex is formed when two or more coordinate positions about the metal ion
- are occupied by donor groups of a single ligand to form an internal ring structure.
In soil role of ligands fulfilment simple organic compounds and functional groups of humic substances.
- The order of decreasing affinity of organic groupings for metal ions is approximately as
- follows:
| -O- | > | -NH2 | > | -N=N- | > | =N | > | -COO- | > | -O- | > | C=O |
| enolate | amine | azo | ring N | carboxylate | ether | carbonyl |
- The order of decreasing ability of metal ions to chelating is as follows:
Fe3+>Cu2+>Ni2+>Co2+>Zn2+>Fe2+>Mn2+
- The complexing ability of humic and fulvic acids results largely from their content of
- oxygen-containing functional groups, such as COOH, phenolic OH and C=O group.
Soil organic constituents form both soluble and insoluble complexes with metal ions and thereby play a dual role in soil.
- Low - molecular - weight compounds (biochemicals, fulvic acids) bring about the
- solubilization of metal ions and affect their transport to plant roots. In contrast, high - molecular - weight compounds (e.g. humic acids) function as a "sink" for polyvalent cations. Natural complexing agents are of considerable importance in weathering processes and in the movement of sesquioxides into the subsoil.
Clay - organic complexes
- The interaction of organic substances with clay has a multitude of consequences that are
- reflested in the physical, chemical and biological properties of the soil matrix.
Several mechanisms are involved in the adsorption of humic substances by clay minerals, the main ones being:
- van der Waals' forces
- bonding by cation bridging
- H - bonding
- adsorption by association with hydrous oxides
- adsorption on interlamellar spaces of clay minerals
Van der Waals' forces
- Van der Waals' forces operate between all molecules, but are rather weak. Essentially,
- these forces result from fluctuations in the electric charge density of individual atoms. An electrically positive fluctuation in one atom tends to produce an elecrically negative fluctuation in a neigh-boring atom and a net attractive forces results. Attractive forces resulting from these fluctuations are every pair of atoms or molecules.
- Adsorption caused by van der Waals' forces can be of considerable importance in the
- adsorption of neutral polar and nonpolar molecules, particulary those which are high in molecular weight.
Bonding by cation bridging
- Since organic anions are normally repelled from negatively charged clay surfaces,
- adsorption of humic and fulvic acids by clay minerals such as montmorillonite occurs only when polyvalent cations are present on the exchange complex.
- Unlike Na+ and K+, polyvalent cations are able to maintain neutrality at the surface by
- neutralizing both the charge on the clay and the acidic functional group of the organic matter (e.g. COO-).
- The main polyvalent cations responsible for the binding of humic and fulvic acids to soil
- clays are Ca2+, Fe3+ and Al3+. The divalent Ca2+ ion doesn't form strong coordination complexes with organic molecules. In contrast Fe3+ and Al3+ form strong coordination complexes with organic compounds.
The polyvalent cations act as a bridge between two charged sites.
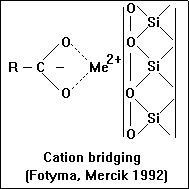
- For a long chain organic molecule, several points of attachment to the clay particle are
- possible.
H - bonding
- This is a linkage between polar groups of the organic molecule and adsorbed water
- molecules or oxygens of the silicate surface through bonding with a single H+ ion.
The strengh of an individual bond is small, but they are additive thus total adsorption energy can be appreciable. Rigorous drying, such as by desication at the soil surface or consumption of available moisture by plant roots, will tend to increase the bonding between humic material and clay by eliminating hydration water and bringing the humic matter in closer contact to the clay.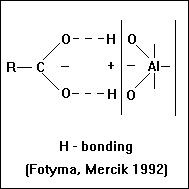
Adsorption by association with hydrous oxides of Fe and Al.
- For many soils, hydrous oxides are equal in importance to mica-type surfaces
in sorbing
- humic substances.
When clay minerals are coated with layers of hydrous oxides their surface reactions are dominated by these oxides rather than the clay.
- Organic anions can be associated with the oxides by simple coulombic attraction. Anion
- associated with clay in this manner are readily removed by increasing the pH or by leaching with NaCl or NH4Cl. The fact that very little humic material can be recovered from soils by these treatments suggest that most of the adsorbed organic matter is retained by supplementary machanisms.
- Coordination or ligand exchange occurs when the anionic group penetrates the
- coordination shell of Al or Fe and becomes icorporated with the surface OH layer. The sorption of fulvic acid on oxide surfaces is accompanied by displacement of OH groups by COO- ions. The organic anion is not easily displaced with simple salts, although adsorption is pH sensitive.
As was the case with organic cations on clay mineral surfaces, a very strong bond will result if more than one group on the humic molecule participates.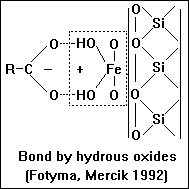
Adsorption on interlamellar spaces of clay minerals
- An important mechanisms for retention of proteins and charged organic cations by
- expandable-layer silicates is through adsorption on interlamellar spaces. Considerably contrversy exist as to whether humic and fulvic acids are bound in this way in the natural soil.
Evidence for interlamellar adsorption of fulvic acid by montmorillonite at pH < 5.5 has been given by Schnitzer and Kodama and Theng. The high - molecular - weight humic acids may be too large to penetrate interlamellar spaces.
Back to first page