- the method leads to the isolation of unaltered material
- the extracted humic substances are free of inorganic contaminants, such as clay and polyvalent cations
- extraction is complete, thereby insuring representation of fractions from the entire molecular-weight range
- the method is universally applicable to all soils
| Type of material | Extractant | Organic matter extracted % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Humic substances | | Mild extractans: | | |||||||||
Alkali extraction
Mild extractans
Na4P2O7 and other neutral salts
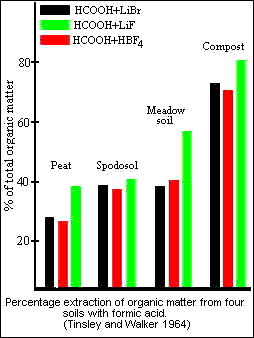
Formic acid - HCOOH
Organic chelating agents
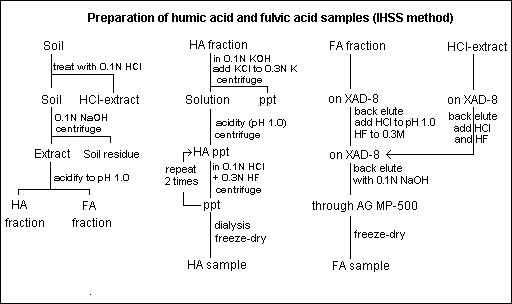
Outline of extraction procedures in IHSS method
Step 18. Combine the final eluates from steps 15 and 17 and pass this solution through XAD-8