1. organic compound of the soil humus,
2. inorganic compounds in which the P is combined with Ca, Mg, Fe, Al and with clay minerals,
3. organic and inorganic P compounds associated with the cells of living matter. Microorganisms are involved in transformations of phosphorus between organic and mineral forms.
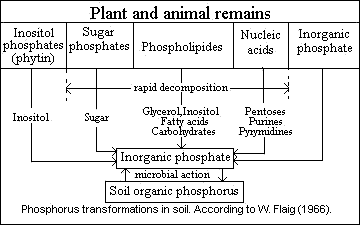
| Inositol phosphates | | Phospholipids | Nucleic acids | Phosphoproteins | Metabolic phosphates | |