Pathway 2 and 3 - The polyphenol theory
In pathway 3 lignin still plays an important role in humus synthesis, but in a different way. In this case phenolic aldehydes and acids released from lignin during microbiological attack undergo enzymatic conversion to quinones, which polymerize in the presence or absence of amino compounds to form humiclike macromolecules.
Pathway 2 is somewhat similar to pathway 3 except that the polyphenols are synthesized by microorganisms from nonlignin C sources (e.g., celulose). The polyphenols are then enzymatically oxidized to quinones and converted to humic substances.As noted earlier, the classical theory of Waksman is now considered obsolete by many investigators. According to current concepts quinones of lignin origin, together with those synthesized by microorganisms, are the major building blocks from which humic substances are formed.
The formation of brown-colored substances by reactions involving quinones is not rare event, but is a well-known phenomenon that takes place in melanine formation, such as in the flesh of ripe fruits and vegetables following mechanical injury and during seed coat formation.
Possible sources of phenols for humus synthesis include lignin, microorganisms, uncombined phenols in plants and tannins.Of these, only the first two have received serious attention.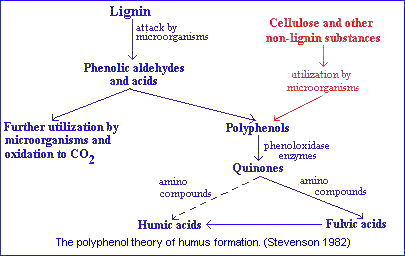
Flaig's concept of humus formation is:- Lignin, freed of its linkage with cellulose during decomposition of plant residues, is subjected to oxidative splitting with the formation of primary structural units (derivatives of phenylpropane).
- The side-chains of the lignin-building units are oxidized, demethylation occurs, and the resulting polyphenols are converted to quinones by polyphenoloxidase enzymes.
- Quinones arising from the lignin (and from other sources) react with N-containing compounds to form dark-colored polymers.
The role of microorganisms as sources of polyphenols has been emphasized by Kononova.She concluded that humic substances were being formed by cellulose-decomposing myxobacteria prior to lignin decomposition.
The stages leading to the formation of humic substances were postulated to be:- Fungi attack simple carbohydrates and parts of the protein and cellulose in the medullary rays, cambrium, and cortex of plants residues.
- Cellulose of the xylem is decomposed by aerobic myxobacteria. Polyphenols synthesized by the myxobacteria are oxidized to quinones by polyphenoloxidase enzymes, and the quinones subsequently react with N compounds to form brown humic substances.
- Lignin is decomposed. Phenols released during decay also serve as source materials for humus synthesis.
Pathway 4 - Sugar-amine condensation
The notion that humus is formed from sugars (pathway 4) dates back to the early days of humus chemistry. According to this concept reducing sugars and amino acids, formed as by-products of microbial metabolism, undergo nonenzymatic polymerization to form brown nitrogenous polymers of the type produced during dehydratation of certain food products at moderate temperatures.
A major objection to this theory is that the reaction proceeds rather slowly at the temperatures found under normal soil conditions. However, drastic and frequent changes in the soil environment (freezing and thawing, wetting and drying), together with the intermixing of reactants with mineral material having catalytic properties, may facilitate condensation. An attractive feature of the theory is that the reactants (sugars, amino acids etc.) are produced in abundance through the activities of microorganisms.
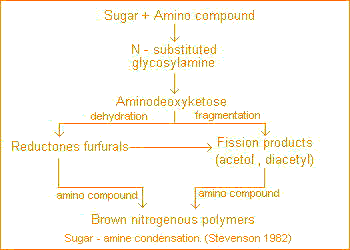 The initial reaction in sugar-amine condensation involves addition of the amine to the aldehyde group of the sugar to form the n-substituted glycosylamine. The glycosylamine subsequently undergoes to form the N-substituted-1-amino-deoxy-2-ketose. This is subject to: fragmentation and formation of 3-carbon chain aldehydes and ketones, such as acetol, diacetyl etc.; dehydration and formation reductones and hydroxymethyl furfurals.
The initial reaction in sugar-amine condensation involves addition of the amine to the aldehyde group of the sugar to form the n-substituted glycosylamine. The glycosylamine subsequently undergoes to form the N-substituted-1-amino-deoxy-2-ketose. This is subject to: fragmentation and formation of 3-carbon chain aldehydes and ketones, such as acetol, diacetyl etc.; dehydration and formation reductones and hydroxymethyl furfurals.
All of these compounds are highly reactive and readily polymerize in the presence of amino compounds to form brown-colored products.
Back to first page
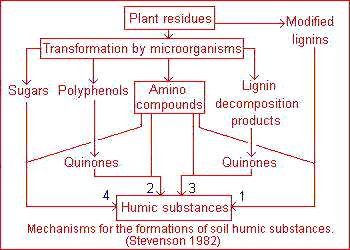 The classical theory, popularized by Waksman, is that humic substances represent modified lignins but the majority of present-day investigators favor a mechanism involving quinones . In practice all four pathways must be considered as likely mechanisms for the synthesis of humic and fulvic acids in nature, including sugar-amine condensation .This four pathways may operate in all soils, but not to the same extent or in the same order of importance. A lignin pathway may predominate in poorly drained soils and wet sediments (swamps, etc.) whereas synthesis from polyphenols may be of considerable importance in certain forest soils. The frequent and sharp fluctuations in temperature, moisture and irradiation in terrestrial surface soils under a harsh continental climate may favor humus synthesis by sugar-amine condensation.
The classical theory, popularized by Waksman, is that humic substances represent modified lignins but the majority of present-day investigators favor a mechanism involving quinones . In practice all four pathways must be considered as likely mechanisms for the synthesis of humic and fulvic acids in nature, including sugar-amine condensation .This four pathways may operate in all soils, but not to the same extent or in the same order of importance. A lignin pathway may predominate in poorly drained soils and wet sediments (swamps, etc.) whereas synthesis from polyphenols may be of considerable importance in certain forest soils. The frequent and sharp fluctuations in temperature, moisture and irradiation in terrestrial surface soils under a harsh continental climate may favor humus synthesis by sugar-amine condensation.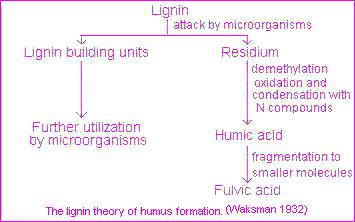 According to this theory, lignin is incompletely utilized by microorganism and the residuum becomes part of the soil humus. Modification in lignin include loss of methoxyl (OCH3) groups with the generation of o-hydroxyphenols and oxidation of aliphatic side chains to form COOH groups. The modified material is subject to further unknown changes to yield first humic acids and then fulvic acids.This pathway, illustrated on the picture , is exemplified by Waksman's lignin-protein theory.
According to this theory, lignin is incompletely utilized by microorganism and the residuum becomes part of the soil humus. Modification in lignin include loss of methoxyl (OCH3) groups with the generation of o-hydroxyphenols and oxidation of aliphatic side chains to form COOH groups. The modified material is subject to further unknown changes to yield first humic acids and then fulvic acids.This pathway, illustrated on the picture , is exemplified by Waksman's lignin-protein theory.
